1976 - Barge AC38
| Year | 1976 |
| Vessel | Barge AC38 |
| Location | Chesapeake Bay, USA |
| Cargo type | Bulk |
| Chemicals | OLEUM (20-65% free SO3) , SULPHURIC ACID (fuming) , SULPHURIC ACID (spent) , SULPHURIC ACID (with more than 51% acid) |
Summary
In the early morning of August 1976, the tug Big Mama was proceeding southwards in Chesapeake Bay, U.S.A. towing a single barge (AC 38). The AC 38 was a 26 year double-skin chemical barge, containing over 1000 tons of 20% oleum. The barge and tug had departed the Allied Chemical facility and was en route to another facility belonging to Allied Chemical.
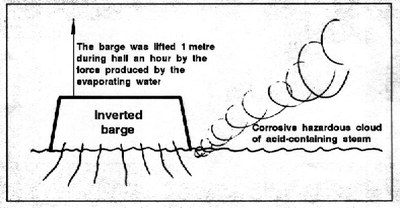
At about 05.40 am, the mate noticed that the hawser (tow rope) was rapidly slewing to port, straining the hawser and causing the tug to heel to port. The mate reacted by stopping the engine. The towing hawser and towing bridle were recovered intact. The tug returned to the barge and found it floating in an inverted position. The tug master called the Coast Guard and stood by the barge awaiting assistance. Some of the crew of the tug noticed a vapour-like smog coming from around the sides of the barge soon after it capsized. This continued for about 10 or 15 minutes. Following this, about 20 minutes after capsizing, the barge suddenly rose up in the water about 1 metre and began to vent great quantities of vapour and steam from underneath (Figure 1). This continued for about 30 minutes. The barge then settled back in the water where it assumed a stable position down by the port bow. This condition persisted until righting two days later on August 20. The Coast Guard boat arrived on the scene and reported seeing dead fish floating on the water surface in the area of the barge and that seagulls were eating these fish without any ill effects.
Oleum is concentrated sulphuric acid: both oleum and sulphuric acid are produced by bubbling sulphur trioxide, a fuming liquid, through water. When all the water present has reacted with sulphur trioxide, the solution is saturated, i.e. the pure 100 per cent acid is formed. By bubbling even more sulphur trioxide, a further molecule of the trioxide is added to form pyrosulphuric acid (H2SO4+SO3(H2S2O7) known also as oleum or fuming sulphuric acid. Further addition of sulphur trioxide produces polysulphuric acid, H2S31O10 up to H2O(SO3)n. The concentration of oleum is classified by percentage (wt) of the excess sulphur trioxide present.
The acid carried on board was 20% oleum (20% by wt sulphur trioxide; 80% by wt sulphuric acid). Twenty percent oleum is equivalent to 104.5% sulphuric acid and the specific gravity of 20% oleum is 1.92. An exothermic process occurs when sulphuric acid is dissolved in water generating substantial amounts of heat. Theoretically, 1000 tons of oleum could cause about 409 tons of water to be heated to steam.
Sulphuric acid is highly corrosive to ferrous metals and produces flammable hydrogen in the reaction process. Corrosion is highest at 28% acid and lowest at 96% acid. Health hazards associated with oleum or sulphuric acid include destruction of eye tissue with attendant loss of vision whilst the vapour or mist from oleum being primarily sulphur trioxide is extremely toxic to the upper respiratory tract. In the formation of vapour mists of oleum on contact with water, studies have shown the vapourization appears to be a combination of direct sulphur trioxide vapourization and vapourization of the acid as a result of violent bubbling from the positive heat of solution. Once vapourized, the small particles would grow by dispersing onto already present dust particles and by hydroscopic action on water vapour in the air. The particles will grow to a maximum size as the hydroscopic action decreases due to dilution and eventually, an acidic mist is formed.
Narrative
In the early morning of August 1976, the tug Big Mama was proceeding southwards in Chesapeake Bay, U.S.A. towing a single barge (AC 38). The AC 38 was a 26 year double-skin chemical barge, containing over 1000 tons of 20% oleum. The barge and tug had departed the Allied Chemical facility and was en route to another facility belonging to Allied Chemical.
At about 05.40 am, the mate noticed that the hawser (tow rope) was rapidly slewing to port, straining the hawser and causing the tug to heel to port. The mate reacted by stopping the engine. The towing hawser and towing bridle were recovered intact. The tug returned to the barge and found it floating in an inverted position. The tug master called the Coast Guard and stood by the barge awaiting assistance. Some of the crew of the tug noticed a vapour-like smog coming from around the sides of the barge soon after it capsized. This continued for about 10 or 15 minutes. Following this, about 20 minutes after capsizing, the barge suddenly rose up in the water about 1 metre and began to vent great quantities of vapour and steam from underneath (Figure 1). This continued for about 30 minutes. The barge then settled back in the water where it assumed a stable position down by the port bow. This condition persisted until righting two days later on August 20. The Coast Guard boat arrived on the scene and reported seeing dead fish floating on the water surface in the area of the barge and that seagulls were eating these fish without any ill effects.
Oleum is concentrated sulphuric acid: both oleum and sulphuric acid are produced by bubbling sulphur trioxide, a fuming liquid, through water. When all the water present has reacted with sulphur trioxide, the solution is saturated, i.e. the pure 100 per cent acid is formed. By bubbling even more sulphur trioxide, a further molecule of the trioxide is added to form pyrosulphuric acid (H2SO4+SO3(H2S2O7) known also as oleum or fuming sulphuric acid. Further addition of sulphur trioxide produces polysulphuric acid, H2S31O10 up to H2O(SO3)n. The concentration of oleum is classified by percentage (wt) of the excess sulphur trioxide present.
The acid carried on board was 20% oleum (20% by wt sulphur trioxide; 80% by wt sulphuric acid). Twenty percent oleum is equivalent to 104.5% sulphuric acid and the specific gravity of 20% oleum is 1.92. An exothermic process occurs when sulphuric acid is dissolved in water generating substantial amounts of heat. Theoretically, 1000 tons of oleum could cause about 409 tons of water to be heated to steam.
Sulphuric acid is highly corrosive to ferrous metals and produces flammable hydrogen in the reaction process. Corrosion is highest at 28% acid and lowest at 96% acid. Health hazards associated with oleum or sulphuric acid include destruction of eye tissue with attendant loss of vision whilst the vapour or mist from oleum being primarily sulphur trioxide is extremely toxic to the upper respiratory tract. In the formation of vapour mists of oleum on contact with water, studies have shown the vapourization appears to be a combination of direct sulphur trioxide vapourization and vapourization of the acid as a result of violent bubbling from the positive heat of solution. Once vapourized, the small particles would grow by dispersing onto already present dust particles and by hydroscopic action on water vapour in the air. The particles will grow to a maximum size as the hydroscopic action decreases due to dilution and eventually, an acidic mist is formed.
Resume
According to the crew, there was no indication that cargo had been released at any time. Based on this information, the Coast Guard organized the personnel and equipment to safely right the capsized barge.
By the morning of August 19, the following were assembled: two 100-ton capacity Navy crane barges; two commercial tugs; a towing vessel; a tug which served as a command post. Technical personnel were provided by the chemical company who assumed financial responsibility.
A diver hired by the salvage master surveyed the submerged portion of the barge. Working under conditions of poor visibility, strong currents and lack of knowledge of the barge, he reported no damage nor did he find anything out of the ordinary.
Assuming that there would be venting of oleum on righting, it was decided that the safest way to righten the barge was for the two most powerful tugs to tow the barge upwind with tow hawsers passed over the bottom of the barge. As the barge was to be pulled broadside through the water, a sufficient righting moment was expected to flip the barge back to the upright position. In the process, all other vessels were to remain upwind whilst one of the tugs was to stand close by, ready to wash down the barge using her fire-fighting monitor nozzles, if a vapour cloud was to form. To ensure that if any cargo vapours were released, it was decided to move the operation 2 miles further offshore. Due to the great difference in horse power, the tugs were not able to keep an equal pulling strain and the barge slewed to one side. After different efforts, including changing the towing arrangements of the tugs, the righting attempts were suspended due to nightfall.
Before anchoring, the barge was moved away from the heavily trafficked maritime routes in Chesapeake Bay. It was also decided to use the 100-ton capacity Navy crane barges to righten the barge the following day.
The two crane barges were lashed together and two heavy wire cables were passed under the barge and around the barge to the towing bits on the near side. One of the wire cables parted on the first righting attempt and was replaced by a chain. Upon righting, there was no indication of any acid-water interaction or vapour cloud production. Onboard inspection of the barge showed that the tanks were full of water rather than acid. There was evidence of recent corrosion around the cargo tank hatches whilst the cargo tank gaskets and seals were missing. Cargo tank tops were deformed inwards indicating that there had been a vacuum inside the tanks. The explanation for this was that when the barge capsized, the load of the heavy liquid bore directly on the inverted tank tops. The structure along with the external framing and tanks tops were being designed for this load and deformed outwards. Where the cargo was released, the tanks were full of steam, acid mist and hot air. A vacuum developed with a resultant deformation of the tank tops inward due to the rapid reduction in pressure caused by cooling and condensation which could not be adequately compensated for by the water drawn in through the open hatches. Unfortunately, the fact that cargo had been released only came to light at the inquiry some weeks later when the cook on the tug was asked if there was anything else he could remember about the incident!