1977 - Storage Tank
| Year | 1977 |
| Vessel | Storage Tank |
| Location | Massie Creek, USA |
| Cargo type | Bulk |
| Chemicals | AMMONIA anhydrous , AMMONIA aqueous solution (28% or less) |
Summary
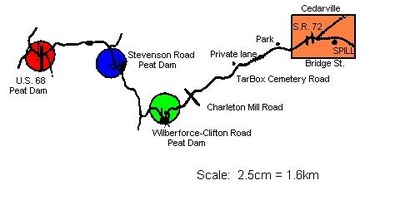
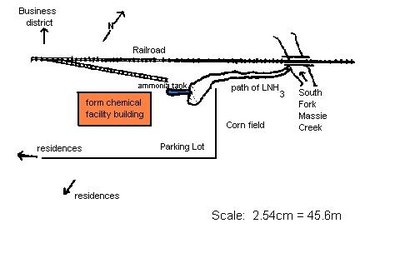
Shortly after 3.00pm on May 2, 1977, a hose failed on a truck transferring liquid ammonia (LNH3) into a 760,000 litre storage tank at a farm chemical service installation in Cedarville, Ohio, U.S.A. The tank was located above ground on a gravelled driveway in the south-eastern section of Cedarville near a railroad spur (Figure 1).
Normal drainage from the facility is poor, but the nominal drainage that exists is towards the South Fork of Massie Creek by means of a grassy wetland along the railroad tracks. The South Fork joins the North Fork of Massie Creek in Cedarville; Massie Creek then flows into the Little Miami River. The river is a state as well as a federally designated scenic river, and as such is one of the important environmental resources in the state of Ohio.
The truck belonged to the tank line company and was used for carrying both LNH3 and LPG. Conversion from one load to another required only different discharge lines. The two types of lines are similar in appearance, however, the LNH3 lines are sturdier, wire reinforced and are clearly labelled for LNH3 use. On this occasion however, the hoses had not been switched, and the weaker LPG hose was used for transfer. The hose soon blew out, trapping the driver, and spreading fumes over the immediate area. The driver was rescued and taken to a nearby hospital and the local fire department summoned to the scene. They determined that it would be necessary to spray water on the leaking hose/area to reduce the potential threat to humans and businesses in the area.
Previous investigations have shown that ammonia spilled onto water surfaces partitions into water and air in about 60/40 ratio.
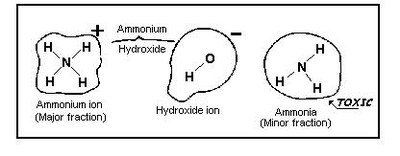
Ammonia is very soluble in water (89.9g/100ml water at 0ºC), forming a chemical equilibrium with water in which non-ionized ammonia, ionized ammonia and hydroxyl ions are present simultaneously (Figure 2). Although the non-ionized ammonia is the minor fraction, it is this fraction which is responsible for the toxic effects in aquatic organisms.
Narrative
Shortly after 3.00pm on May 2, 1977, a hose failed on a truck transferring liquid ammonia (LNH3) into a 760,000 litre storage tank at a farm chemical service installation in Cedarville, Ohio, U.S.A. The tank was located above ground on a gravelled driveway in the south-eastern section of Cedarville near a railroad spur (Figure 1).
Normal drainage from the facility is poor, but the nominal drainage that exists is towards the South Fork of Massie Creek by means of a grassy wetland along the railroad tracks. The South Fork joins the North Fork of Massie Creek in Cedarville; Massie Creek then flows into the Little Miami River. The river is a state as well as a federally designated scenic river, and as such is one of the important environmental resources in the state of Ohio.
The truck belonged to the tank line company and was used for carrying both LNH3 and LPG. Conversion from one load to another required only different discharge lines. The two types of lines are similar in appearance, however, the LNH3 lines are sturdier, wire reinforced and are clearly labelled for LNH3 use. On this occasion however, the hoses had not been switched, and the weaker LPG hose was used for transfer. The hose soon blew out, trapping the driver, and spreading fumes over the immediate area. The driver was rescued and taken to a nearby hospital and the local fire department summoned to the scene. They determined that it would be necessary to spray water on the leaking hose/area to reduce the potential threat to humans and businesses in the area.
Previous investigations have shown that ammonia spilled onto water surfaces partitions into water and air in about 60/40 ratio.
Ammonia is very soluble in water (89.9g/100ml water at 0ºC), forming a chemical equilibrium with water in which non-ionized ammonia, ionized ammonia and hydroxyl ions are present simultaneously (Figure 2). Although the non-ionized ammonia is the minor fraction, it is this fraction which is responsible for the toxic effects in aquatic organisms.
Resume
An unknown amount of water was sprayed on the fumes. About an hour later, it was thought that the spill was over; most of the fumes had dissipated and there was no further need for alarm and no significant environmental damage. The spill size was also estimated to be very small. This information later proved to be erroneous, delayed significant actions for clean-up by 24 hours, and made destruction of the aquatic ecosystem in the Massie Creek stream system inevitable. State officials were notified of the spill at about 4.30pm, being told that no ammonia had reached the South Fork of Massie Creek or any other body of water.
Investigation by the Ohio Environment Protection Agency (EPA) early the next morning proved that the report was totally misleading. At 8.00am it was learned that instead of a small ammonia spill, the actual quantity lost amounted to about 16,000 litres, nearly the entire truckload. Puddles of liquid resulting from the spraying by the fire department remained around the tank area whilst vegetative destruction along the grassy low-lying wetland was evident and it soon become apparent that the water from the fire hoses had forced the ammonia into a solution which flowed down the grassy low-lying wetland into the South Fork of Massie Creek. The puddles had an overpowering eye-watering ammonia odour.
Water intake by Cedarville at Bridge Street to an upground was stopped and was to remain closed for about a week.
There were dead fish immediately below confluence of the grass wetland where this met the stream and, progressively, fishkills were observed further downstream. By 3.45pm, all fish were dead at Charleton Mill Road (Figure 3).
At this time, Ohio EPA personnel decided that actions would be taken to try to stop the fishkill and especially to try to prevent an adverse impact on the Little Miami River, if indeed that was possible.
The most logical approach seemed to be based on the pH related toxicity characteristics of ammonia. If stream pH were lowered, phytotoxicity would be reduced ten fold for each decrease of one pH unit. The first action was to install peat moss dams in the stream. Peat moss was chosen because it: a) helps lower and buffer the stream pH to some extent; b) can absorb some ammonia; and c) it was immediately available at local garden and discount stores. The peat moss dams were to be installed below the frontal edge of the spill slug.
By 6.00pm, a peat moss dam had been installed in Massie Creek at Wilberforce-Clifton Road. The dam consisted of: a) fence post supports driven into the streambed; b) chicken wire attached to the posts; c) bales of straw immediately behind the wire; and d) peat moss spread loosely behind the bales. About 15 bales of peat moss (0.3m³) were placed behind the dam structure.
The kill progressed and it was decided that a second phase of toxicity modification would be necessary, again based on pH versus ammonia toxicity. This was to be accomplished by adding hydrochloric acid to the stream. Hydrochloric acid was chosen because: a) it was readily available; b) it is relatively inexpensive; c) it could lower stream pH significantly; and d) it would chemically combine with part of the ammonium ions to form ammonium chloride which can also reduce stream pH and is less toxic than ammonia. It was also decided that additional peat moss dams would be needed at Stevenson Road and at U.S. 68. In total, 56 bales of peat moss, assorted fence posts, chicken wire, and about two dozen bales of straw were used to construct the three peat moss dams which were completed before midnight. All three dams were installed in moderate stream depth conditions (about 0.3 - 0.6 metres) in areas with good flow and lateral mixing stream characteristics.
Additional company personnel arrived and, shortly after midnight, nine drums (55 gallons) of hydrochloric acid were delivered. The acid was added to Massie Creek throughout the night under Ohio EPA supervision: drums of acid were manually poured and siphoned into Massie Creek at Wilberforce-Clifton Road (three drums at 4.00am), at Charleton Mill Road (one drum at 5.00am), and (three drums) at Stevenson Road. Fumes resulting from the addition of acid to the stream were very irritating; in retrospect, self-contained breathing apparatus should have been used. Additional acid arrived from a chemical plant at 6.00am at highway U.S. 68. Highway U.S. 68 was chosen as the main place for acid addition, because it was only 1/2km from the main river and was easily accessible.
At about 6.30am, when the ammonia reached toxic levels at highway U.S. 68, addition of HCl to the stream began at that site. Drums of acid were poured down the streambank below the bridge and above the peat moss dam. The drum dumping site was checked for proper stream mixing by the use of rhodamine dye. The dye was dumped into the stream in a slackwater area with sufficient current to allow for a gradual but steady mixing with the main streamflow in Massie Creek. There was some boiling of water in the immediate drum dump area, but no problems other than irritating fumes. Flow patterns were carefully watched to determine mixing patterns.
A total of 106 fifty-seven-litre drums of acid were added to the stream at highway U.S. 68 over a 5 - 6 hour period. This action reduced stream pH by 1 full unit, thereby reducing ammonia toxicity by at least tenfold.
In total, 7,900 litres of hydrochloric acid were added to Massie Creek. All fish life and nearly all other aquatic life were killed above the point of acid addition at highway U.S. 68, but the fish in the last half kilometer of Massie Creek below highway U.S. 68 were spared and subsequent monitoring of the Little Miami River showed no adverse impact on that stream and no reported dead fish. Additional samples of the stream taken above and below the peat moss dam at highway U.S. 68 showed that: a) the peat absorbed some ammonia; and b) it lowered stream pH levels slightly.
The only other action requested by the Ohio EPA was that of physically removing the dead fish that had accumulated near the Cedarville Water intake in Cedarville and which began to decay. The dead fish would have resulted in foul tasting water.
An analysis of the events leading to the accident as well as an analysis of the response efforts concluded that:
a) using the proper equipment when handling or off-loading hazardous materials is imperative to prevent accidents;
b) a critical factor was that initial reports were misleading and delayed response;
c) the grassy wetland near the spill could have been diked and the contaminated liquid pumped into trucks and/or tank cars for disposal. This would have prevented ammonia solution from reaching the water;
d) faster response could have prevented the spilled acid pulse to reach points further downstream;
e) the hydrochloric acid and peat moss dams were very successful and effective although appropriate breathing apparatus should have been used by response personnel when treating the stream.
f) it is possible to treat and/or alter ammonia once spilled into an aquatic environment.