1979 - Sinbad
| Year | 1979 |
| Vessel | Sinbad |
| Location | Off Ijmuiden, Netherlands |
| Cargo type | Package |
| Chemicals | CHLORINE |
Summary
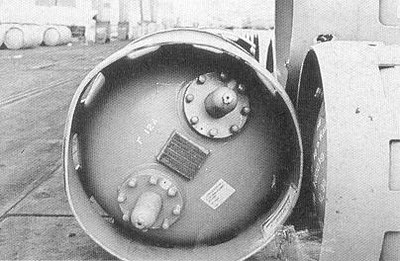
On December 10, 1979, the M/V Sinbad lost most of her deck cargo which included 51 chlorine cylinders, each containing 1,000kgs of chlorine under 2-6 bar pressure depending on the ambient temperature. The location of the loss was only roughly established at approximately 20 miles west of Ijmuiden, the Netherlands, the entrance to the port of Amsterdam. The depth of the water in the area is of 25 to 30 metres. The presence of the lost cylinders was considered a threat to fishermen who might tamper with the containers once they caught them in their nets, as well as to the shipping and the environment once they corroded and leaked. It was decided to start to locate the cylinders, retrieve them and hand them over to Akzo Salt Chemical Industries which would deal with them. This was the only firm in the Netherlands which could test the cylinders and empty them. The chlorine cylinders lost were made of steel and were of a standard type with protruding rims at both ends. At one end, there are openings which are closed by a cover plate held in place with bolts. A valve is fitted which is protected by a cap (Figure 1). Around the cylinder there are two rolling bands for handling. The colour of the container is yellow with a diameter of 90cm and approximately 2 metres in length. Each container is marked with an identification plate containing details of the testing of the container. When filled, they hold approximately 820 litres of liquid chlorine.
The initial action involved a search of the area, split up into two search areas of 18.5kms x 1km, which was determined by the ship positioning, course, speed and hydro-meteo information. In January 1980, five cylinders were located by a concerted search action of vessels belonging to the North Sea Directorate and minesweepers of the Royal Netherlands Navy. The cylinders were attached to steel lines by divers and recovered. Procedures with harbour authorities had to be arranged for landing these containers and transporting them to the chemical facility for disposal.
The North Sea Directorate tried to encourage fishermen in the recovery of the other lost containers by offering financial remuneration for each container brought ashore, but other authorities were reluctant with this approach. As no more chlorine containers were found, as well as due to stormy weather periods and other commitments, the search was abandoned by the authorities.
During the following four years, seven cylinders were caught in nets of fishermen. The last three found in August 1984 were severely corroded. The poor state of the cylinders was felt to constitute a threat to fishermen and other seafarers. An elaborate response strategy was subsequently planned by the Dutch authorities to deal with the situation.
Narrative
On December 10, 1979, the M/V Sinbad lost most of her deck cargo which included 51 chlorine cylinders, each containing 1,000kgs of chlorine under 2-6 bar pressure depending on the ambient temperature. The location of the loss was only roughly established at approximately 20 miles west of Ijmuiden, the Netherlands, the entrance to the port of Amsterdam. The depth of the water in the area is of 25 to 30 metres. The presence of the lost cylinders was considered a threat to fishermen who might tamper with the containers once they caught them in their nets, as well as to the shipping and the environment once they corroded and leaked. It was decided to start to locate the cylinders, retrieve them and hand them over to Akzo Salt Chemical Industries which would deal with them. This was the only firm in the Netherlands which could test the cylinders and empty them. The chlorine cylinders lost were made of steel and were of a standard type with protruding rims at both ends. At one end, there are openings which are closed by a cover plate held in place with bolts. A valve is fitted which is protected by a cap (Figure 1). Around the cylinder there are two rolling bands for handling. The colour of the container is yellow with a diameter of 90cm and approximately 2 metres in length. Each container is marked with an identification plate containing details of the testing of the container. When filled, they hold approximately 820 litres of liquid chlorine.
The initial action involved a search of the area, split up into two search areas of 18.5kms x 1km, which was determined by the ship positioning, course, speed and hydro-meteo information. In January 1980, five cylinders were located by a concerted search action of vessels belonging to the North Sea Directorate and minesweepers of the Royal Netherlands Navy. The cylinders were attached to steel lines by divers and recovered. Procedures with harbour authorities had to be arranged for landing these containers and transporting them to the chemical facility for disposal.
The North Sea Directorate tried to encourage fishermen in the recovery of the other lost containers by offering financial remuneration for each container brought ashore, but other authorities were reluctant with this approach. As no more chlorine containers were found, as well as due to stormy weather periods and other commitments, the search was abandoned by the authorities.
During the following four years, seven cylinders were caught in nets of fishermen. The last three found in August 1984 were severely corroded. The poor state of the cylinders was felt to constitute a threat to fishermen and other seafarers. An elaborate response strategy was subsequently planned by the Dutch authorities to deal with the situation.
Resume
After the retrieval of the highly corroded cylinders in August 1984, assessments were made of how long it was likely to be before the remaining cylinders on the seabed developed leaks (Figure 3). It was estimated that these containers would probably have a residual corrosion life of 2.5 years, after which time they would pose a significant threat to passing ships and fishing boats. On balance, it was considered that the environmental hazards were of secondary importance since, although chlorine reacts slightly with sea water, it is not persistent, carcinogenic or bioaccumulative. Those most at risk from the potential leakage of chlorine gas were thought to be: 1) the crews of fishing boats which might inadvertently haul in one of the containers in their fishing nets; and 2) the crews and passengers of ships and yachts passing through the area.
Further calculations were carried out to assess the hazards posed to nearby coastal communities. It was assumed that should a cylinder on the seabed begin to leak spontaneously it would rise to the surface when 1/3 of its contents had escaped. On reaching the surface, the remaining contents would be released with a rapid expansion of vapour. It was clear from the calculations that, even under the worst conditions, such an escape of chlorine gas would not endanger the coastal communities in the area.
In view of the corroded condition of the cylinders found on the seabed, Akzo Salt Chemicals had advised that a way should be found to deal with the problem at sea. Several alternative methods were investigated and tested, such as:
- transferring the chlorine in the cylinders to new containers on board the response vessel via connecting valves. This alternative was rejected because, after over four years on the seabed, the cylinders were expected to be in a critical condition and possibly unable to deal with the change in external hydrostatic pressure;
- emptying the cylinders via a new valve inserted directly into the container wall using the so-called "hot-tap" method. Apart from the fact that such a valve was not available for chlorine, this approach was also rejected because of the risk of starting an iron oxide fire which could have burned away part of the cylinders;
- puncturing the cylinders to allow the chlorine to evaporate in a controlled manner. Estimates showed that it would have taken several hours before all the contents would have been released. Moreover, once the cylinders would have become buoyant and start to rise to the water surface, after releasing part of their contents, it would have been very difficult to control a direct air release of the chlorine remaining in the cylinders. As a result of these drawbacks, this method was disregarded.
After reviewing all the potential risks involved, it was decided that further efforts should be made to locate the remaining cylinders and destroy them on site under controlled conditions using explosives.
The operation involved the following stages:
- localizing and inspecting the cylinders;
- freeing the cylinders;
- moving the cylinders;
- clearing the area of shipping;
- attaching an explosive charge;
- releasing ammonia to make the cloud visible;
- carrying out a controlled explosion of the cylinders;
- salvaging the empty cylinders.
Many problems were encountered in locating the cylinders, such as:
- the general uncertainty about the course the vessel was following when the incident happened and where exactly along this route the cargo was lost;
- long periods of stormy weather;
- lack of funds;
- long search operations without localizing cylinders;
- the difficulty in fixing the exact position of cylinders recovered by fishermen because of the wide area covered by their net trawls.
Most of the chlorine cylinders had become almost completely embedded in the sand and as a result had to be freed by water jets and pulled out by steel lines.
For safety reasons, the cylinders had to be moved about 100 metres away from the location where they were found.
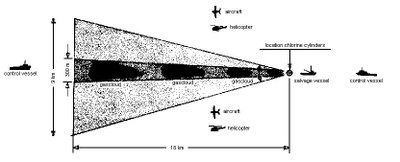
The effect of exposure to chlorine is determined by two main factors: 1) concentration of the substance in the air; and 2) length of exposure. Computer models were used to estimate how large an area needed to be kept free of shipping during the time the cylinders were being destroyed. Since the density of chlorine is three times that of air, a dense-cloud model was used for this purpose. Worst-case predictions were prepared on the basis of an instantaneous release, under the influence of various wind speeds. This was done to investigate the movement of a chlorine gas cloud under different weather conditions.
On the basis of these calculations and a subsequent field trial to confirm the output of the model, it was concluded that a triangular shaped exclusion zone of approximately 18 kilometres length extending to a maximum width of 9 kilometres would be needed to safeguard shipping downwind of the detonation point. An emergency craft was located some 20 miles downwind to monitor this safety zone.
To ensure that the destruction was instantaneous, an explosive charge of approximately 10kgs Donarit S was attached to each container under the skirt on the sides of the valves. The charges were positioned during still water period at a time when the current in the area was at a minimum.
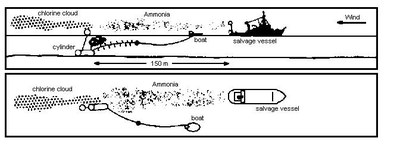
From ammonia cylinders at the upper rear deck of the response vessel, a continuous stream of ammonia was released to help in the visualization of the chlorine cloud. The ammonia gas was released from the control vessel about 150m upwind from the detonation location to make the chlorine-gas cloud visible.
Although a cloud of chlorine gas initially has an intensive grass-green colour, it only remains visible for a period of about 25 seconds or after the cloud has travelled some 200 metres. The presence of the ammonia meant that the colour of the chlorine gas cloud changed from greenish yellow to yellowish white due to the formation of ammonium chloride. This allowed the path of the cloud to be tracked by aircraft (Figure 4) for about 10 - 20 minutes.
At the moment of explosion, the whole area was free from shipping. The area was controlled from the air by a fixed wing aircraft, a helicopter and other support vessels. The on-scene co-ordinator, assisted by industry representatives, gave the go-ahead signal to the diving team who were located at a safe distance. Within 3 seconds, the cloud had emerged from the sea. The cloud drifted as a whole with the wind and lifted shortly after emission above the sea level. There was no visible contact between the sea surface and the cloud. From the ship, the cloud could be followed due to the release of ammonia. The sequence of events from destruction to the addition of ammonia is shown in Figure 5.
When a group of cylinders had to be destroyed, an interval of five minutes was maintained between explosions to ensure that none was omitted. During the entire operation, all those involved wore protective clothing and self-contained breathing apparatus.
To avoid empty cylinders being picked up on the side-scan sonar equipment in further search operations, the remaining fragments of the containers were removed from the seabed. An added advantage of collecting the empty shells was that it allowed the extent of corrosion to be determined.
Following the operation, eight cylinders were still unaccounted for and due to the highly corroded state of the containers, the concern was that the slightest impact could release the entire contents. Results of a risk analysis showed that:
- the eight cylinders that still remained on the seabed constituted an acceptable risk to shipping in the area taking into account the fact that vessels could minimize the hazards involved by setting a course perpendicular to the wind direction as soon as a chlorine odour is detected;
- fishermen constituted the group at highest risk, being the people most likely to experience an instantaneous release of chlorine gas when hauling corroded cylinders in their nets.